OPTIMOX ATP COFACTORS INFORMATION
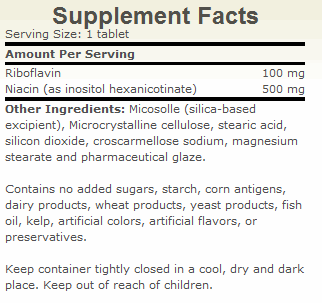
| The Key To Managing Fibromyalgia and Thyroid Health By Guy E. Abraham, MD Fibromyalgia (FM) is a common clinical syndrome of generalized musculoskeletal pain, stiffness and chronic aching, characterized by reproducible tenderness on palpation of specific anatomical sites, called tender points. This condition is considered primary when not associated with systemic causes, trauma, cancer, thyroid diseases and pathologies of rheumatic or connective tissues.1 FM is now recognized as being one of the most common rheumatic complaints with clinical prevalence of 6-20 percent.2 This syndrome is predominantly observed in middle-aged women (30-50 years old), and to a lesser degree in men. Community based studies showed a prevalence of 2 percent of the general population. 3 Epidemiological studies revealed that up to 6 percent of school age children fulfill the criteria for FM. 4-5 Chronic fatigue is a common complaint in FM patients6, causing severe disability. The negative impact of FM symptomatology on the quality of life, the workforce and healthcare cost7 warrants a careful search for the pathophysiology involved and consequently for an effective treatment program, based on a clear understanding of the mechanism involved in this morbid condition. Proposed Etiologies of FM More than 100 years ago, inflammatory reaction was proposed by Stockman8 as the cause of “Chronic Rheumatism.” However, this has not been confirmed by histologic examination (the study of the microscopic structure of tissue).9 A multi-factorial etiology, with stress being the common pathway, has been proposed.10 Elevated catecholamines are observed in urine of FM patients.11 However, anxiolytic (anxiety-reducing) agents are of limited therapeutic values.12-13 Tryptophan-serotonin deficiency was suggested as a possible causative factor in the myalgia of FM patients.14 Although plasma free tryptophan levels correlated inversely with the severity of muscle pain in FM patients, the oral administration of tryptophan to FM patients actually worsened musculoskeletal pain.15 Depriving normal college students of stage IV sleep resulted in musculoskeletal pain similar to the myalgia observed in FM patients.16 However, sleep disturbance is not the cause of FM because ingestion of tryptophan improved sleep pattern but worsened muscle pain.16 Eisenger et al.17 who did extensive clinical and laboratory studies on FM patients, reported several abnormalities in carbohydrate metabolism and decreased ATP levels. He stated: “Thus, we conclude that the pain experienced by FM patients is related in part to biochemical lesions and that this condition requires appropriate metabolic therapy rather than the traditional approach with analgesics and antidepressants.” Essentially, Eisenger is recommending a nutritional approach to FM instead of symptomatic relief with prescription drugs. Of all the proposed etiologies of FM, the one that fits best the available data is chronic hypoxia18 and decreased ATP synthesis.19 Patients with FM have normal muscle blood flow under resting conditions, but decreased blood flow under aerobic exercises.20 Muscle tissue oxygen pressure is significantly lower than in normal controls in subcutaneous tissue of FM patients,21 suggesting that the hypoxic condition is not limited to the tender muscles although the hypoxia is more severe at tender points. Low levels of high energy phosphate such as ATP, ADP and phosphocreatine were observed at tender points, together with increased AMP levels.22 The levels of high energy phosphates were significantly lower in tender muscles than in non-tender muscles of FM patients and in muscles of normal controls. Red blood cell ATP levels are lower in FM patients than in normal subjects.23 Enhancing ATP Synthesis The synthesis of ATP by intact respiring mitochondria requires the presence of oxygen, magnesium, substrate, ADP and inorganic phosphate.19 When all substances are present in optimal concentration, the integrity of the mitochondrial membrane and the capacity of enzymatic system in the respiratory chain becomes rate limiting. We have previously discussed the importance of the 5 ingredients required for ATP synthesis and the central role of malate, obtained from food sources and from synthesis in the citric acid cycle.19 Magnesium and the B vitamins are required in adequate amounts for the normal metabolism of carbohydrates and other macronutrients in the citric acid cycle. The turnover of ATP is extremely high. For example, a human at rest consumes one half of his/her weight of ATP daily. The synthesis of ATP from ADP plus a high-energy phosphate group is called oxidative phosphorilation and is dependent on the electron flow through the electron transport chain via electron carriers. Two of the B vitamins are directly involved in ATP synthesis: riboflavin and niacin. NADH and FADH2 are the major electron carriers in the synthesis of ATP. The B vitamins, niacin and riboflavin, are the precursors of the cofactors NADH and FADH2. These cofactors play an important role also in the oxidation and organification of iodide by generating hydrogen peroxide via the NADPH oxydase system.24 In some conditions, the body cannot efficiently synthesize NADH and FADH2 from niacin and riboflavin because of defect/damage to the enzymes involved in this conversion.25-28 More riboflavin and niacin are needed to override the inefficient enzymes in order to obtain adequate levels of Cofactors. Preliminary results suggest that high dosage of vitamins B2 and B3 (ATP Cofactors) combined with 100 mg of elemental iodine in the form of Lugol tablets resulted in a significant improvement of overall wellbeing in FM subjects above the response observed with iodine alone.24,29 We have tested increasing amount of riboflavin and niacin in FM patients. Niacinamide and inositol hexanicotinate were used as non-flushing derivatives of niacin. Beneficial effects were not observed until the daily intake reached 200 mg riboflavin and 1,000 mg niacin. Symptomatic improvement of patients with autoimmune thyroiditis was reported by Brownstein following orthoiodosupplementation with the ATP Cofactors.30 Decreased TPO antibody titers were observed in all the FM patients with elevated titers29 and also in patients with autoimmune thyroiditis30 following the combination of orthoiodosupplementation with ATP Cofactors. ATP’s Widespread Role in Health Eisenger et al.,17 who performed extensive clinical and laboratory studies in FM patients, concluded that the nutritional approach to correct underlying metabolic abnormalities was preferable to symptomatic treatment. Enhancing ATP synthesis seems to be the key to the management of FM patients. As a side benefit, the same ATP Cofactors involved in ATP synthesis are also involved in generating H2O2 in target organs possessing proxydases, not just the thyroid gland. The generation of hydrogen peroxide is the rate-limiting step in the organification of iodide, not just for the synthesis of thyroid hormones, but also in the protection of cell membrane lipids from oxidative damage, and the response of target organs to steroid hormones31 including calcitriol, (1, 25-OH-D3), which is a steroid hormone, synthesized from vitamin D. Calcitriol promotes the absorption of calcium and phosphate in the intestinal track and their deposition in bone tissue. Increased organification of iodide in the thyroid gland following ATP Cofactor was observed in FM patients. In Table 1 are displayed the results of thyroid function tests in 5 FM patients before intervention, after 100 mg iodine for 6 weeks and after the addition of the ATP Cofactors for another 6 weeks. The mean values of all thyroid hormones measured decreased following 6 weeks on iodine at 100 mg/day. However, all mean values increased to baseline levels following 6 weeks on ATP Cofactors. Conclusion Orthoiodosupplementation with ATP Cofactors should be part of a complete nutritional program emphasizing magnesium instead of calcium. In the author’s experience, megadosing with calcium (2,000-3,000 mg/day) has been the most common cause of poor response to orthoiodosupplementation. Physicians and other health care professionals need to be informed about the toxicity of excess calcium,32 and the importance of adequate magnesium intake32-36 for optimal health and strong bones. References 1. Wolf et al. The American College of Rheumatology, 1990 criteria for the classification of fibromyalgia. Arther Rheum. 1990; 33: 160-72. 2. Bennett RM. Fibrositis: Evolution of an Enigma. J Rheumat. 1986; 13:676-678. 3. Wolfe F, et al. The Prevalence and Characteristics of Fibromyalgia in the General Population. Arthritis Rheum. 1995; 38:19-28 4. Buskila D, Press J, et al. Assessment of Nonarticular Tenderness and Prevalence of Fibromyalgia in Children. J Rheumatol. 1993; 20:368-370. 5. Mikkelson M. One Year Outcome Preadolescents with Fibromyalgia. J Rheumatol. 1999; 26:674- 682. 6. Goldenberg DL, et al. High Frequency of Fibromyalgia in Patients with Chronic Fatigue Seen in a Primary Care Practice. Arthr Rheum. 1990; 33:381-387. 7. Gill JM. Fibromyalgia and Diffuse Myalgia – Clinics in Family Practice. 2005; 7:181-190. 8. Stockman R. The Cause, Pathology and Treatment of Chronic Rheumatism. Edin Med J. 1904; 15: 107-116. 9. Yunus MB, et al. Primary Fibromyalgia Syndrome and Myofacial Pain Syndrome: Clinical Features and Muscle Pathology. Arch Phys med Rehabil. 1988; 69:451-454. 10. Daily PA, et al. Psychological Stress and the Fibrositis/Fibromyalgia Syndrome. J Rheum. 1990; 1380-1385. 11. Russell JI. Neurohormonal Aspects of Fibromyalgia Syndrome. Rheum Dis Clinic N Am. 1989; 15: 149-168. 12. Wolfe F. The Clinical Syndrome of Fibrositis. American Journal of Medicin. 1986; 81:7-14. 13. Russell JI, et al. Treatment of Primary Fibrositis/Fibromyalgia Syndrome with Ibuprofen and Alprazolam. Arthr Rheum. 1991; 34:552-560. 14. Moldofsky H, et al. Plasma Tryptophan and Musculoskeletal Pain in Non-Articular Rheumatism (“Fibrositis Syndrome”). Pain. 1978; 5:65-71. 15. Moldofsky H, et al. The Relationship of Alpha and Delta EEG Frequencies to Pain and Mood in “Fibrositis” Patients Treated with Chlorpromazine and L-Tryptophan. Electroencephalogram Clinic of Neurophysiology. 1980; 50:71-80. 16. Moldofsky H, et al. Inductin of Neurasthenic Musculoskeletal Pain Syndrome by Selective Sleep Stage Deprivation. Psychosom Med. 1976; 38:34-44. 17. Eisinger J, et al. Glycolysis Abnormalities in Fibromyalgia. J of the Amer College of Nut. 1994; 13: 144-148. 18. Fassbender HG, et al. Morphologie und Pathegenee des Weichteilrhumatismus. Z Rheymaforschg. 1973; 32:355. 19. Abraham GE, Flechas JD. Management of Fibromyalgia: Rationale for the Use of Magnesium and Malic Acid. J Nut Med. 1992; 3:49-59. 20. Bennett RM, et al. Aerofitness in Paitents with Fibrositis. Arthr Rheum. 1989; 4:454-460. 21. Lund N, et al. Muscle Tissue Oxygen pressure in Primary Fibromyalgia. Scand J Rheumatism. 1986; 15:165-173. 22. Bengtsson A, et al. Reduced high-Energy Phosphate Levels in the Painful Muscles of Patients with Primary Fibromyalgia. Arthr Rheum. 1986; 20:817-821. 23. Russell IJ, Vipraio GA, Abraham GE. Red Cell Nucleotide Abnormalities in Fibromyalgia Syndrome. Arthritis Rheum. 1993; 36:S223. 24. Abraham GE, Flechas JD. Evidence of Defective Cellular Oxidation and Organification of Iodide in a Female with Fibromyalgia and Chronic Fatigue. The Original Internist. 2007; 14:77-82. 25. Figueiredo, Marcia DL, et al. Goiter and Hypothyroidism in Two Siblings Due to Impaired Ca+NAD (P)H Dependant H2O2 Generating Activities. J Clin Endocrinol Metab. 2001; 86:4843-4848. 26. Niempominiszcze H. Abnormal H2O2 Supply in the Thyroid of a patient with Goiter and Iodine Organification Defect. J Clin Endocr Metab. 1987; 65:344-348. 27. Moreno JC, et al. Inactivating Mutations in the Gene for Thyroid Oxydase 2 (THOX2) and Congeniatal Hypothyroidism. N England J Med. 2002; v. 347. 28. Kusakabe T. Deficient Cytochrome B5 Reductase Activity in Nontoxic Goiter with Iodide Organification Defect. Megabolism. 1975; 24:1103-1113. 29. Abraham GE, Flechas JD. The effect of daily ingestion on 100mg iodine in a tablet form of Lugol solution (Iodoral®) combined with high doses of vitamins B-2 and B3 (ATP Cofactors) on various clinical and laboratory parameters in 5 subjects with Fibromyalgia. J. Appl. Nut, In Press 2008. 30. Brownstein D. Iodine: Why You Need It, Why You Can’t Live Without It. Medical Alternative Press, West Bloomfield, MI, 2008. 31. Abraham GE, et al. Orthoiodosupplementation Iodine Sufficiency of the Whole Human Body. The Original Internist. 2002; 9:30-41. 32. Abraham GE. The Calcium Controversy. J Appl Nut. 1982; 34:69. 33. Abraham E. Nutritional Factors in the Etiology of the Premenstrual Tension Syndromes. J Rep Med. 1983; 24:446. 34. Abraham GE, et al. Serum and Red Cell magnesium Levels in Patients with Premenstrual Tension. Am J Clin. Nut. 1981; 34:2364. 35. Abraham GE. The Effects of Nutrients on Premenstrual Mood Changes. In Nutrients and Brain Function, W.B. Essman (Ed), Karger Publishing, Basel, Switzerland, 1987, pp163 36. Abraham GE. The Importance of Magnesium in the Management of Primary Postmenopausal Osteoporosis. J Nut Med. 1991; 2:165-178. |
Back to order page |